Directives and regulations of the European Union are designed to facilitate the free movement of goods and services within the European single market. In the perception of European microtechnology organizations, the impeding effects of EU regulations clearly outweigh the facilitating effects. Particularly SMEs feel overburdened with the documentation and reporting obligations.
In the course of its annual economic data survey in February 2018, the IVAM Microtechnology Network asked microtechnology companies and research institutes in Europe about the practical experiences they made with EU regulations, and the opportunities to exert influence on decision making processes.
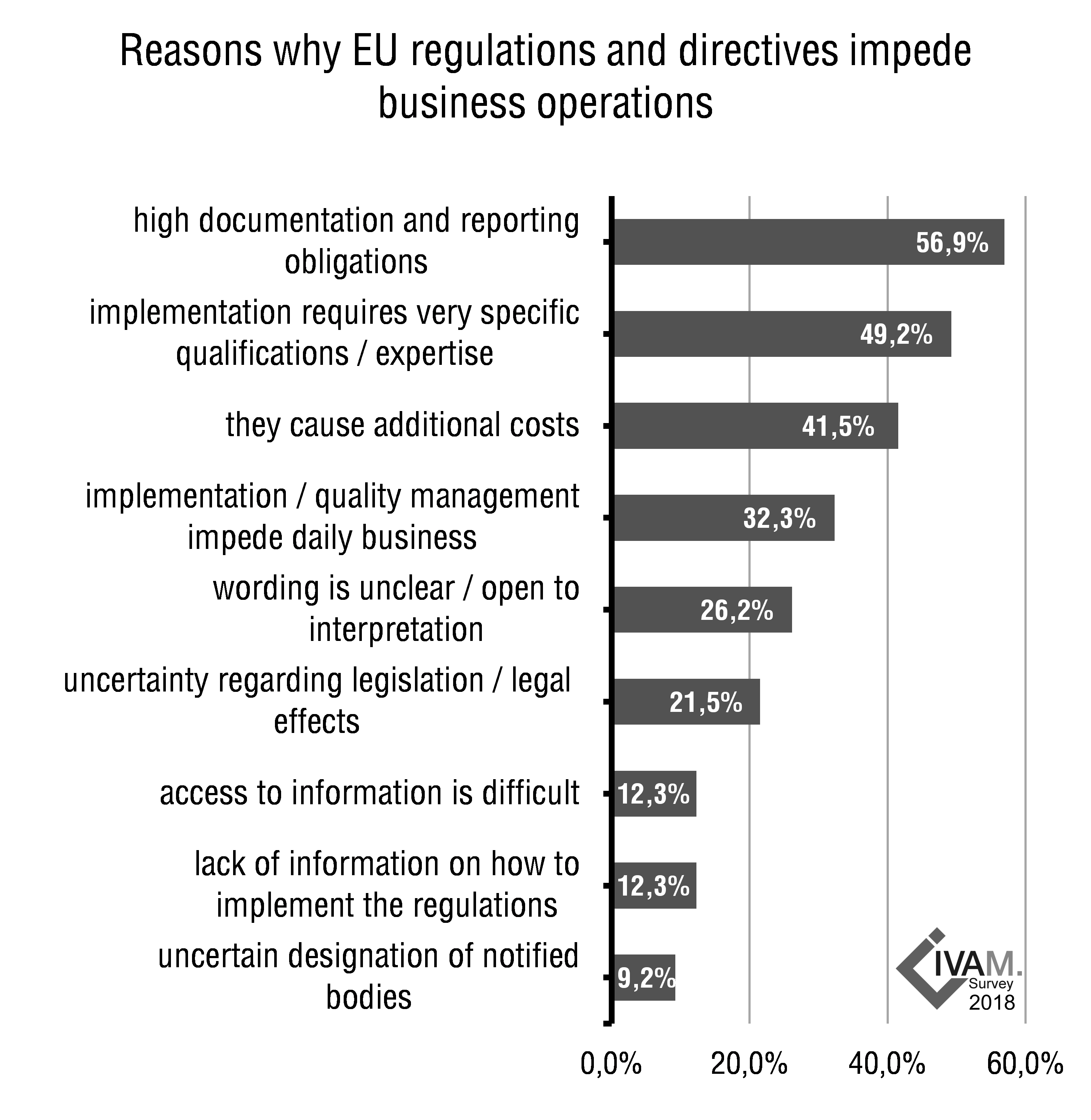
Almost sixty percent of the responding organizations feel that EU regulations and directives are impeding their business operations. A major complication from the point of view of the microtechnology industry representatives are the high documentation and reporting obligations that EU regulations entail. More than half of the companies and institutes say that these obligations impede their business operations. Almost three quarters of organizations call for simplified regulations for SMEs.
The European microtechnology industry is affected by a variety of EU regulations and directives, such as RoHS (Restriction of Hazardous Substances), REACH (Registration Evaluation, Authorization and Restriction of Chemicals), or the Machinery Directive. Recently, regulations that apply to device manufacturers have become relevant for the supplier industry as well. The new Medical Device Regulation, for instance, which came into force in May 2017, has considerably increased the requirements of certification and documentation for suppliers of the medical device industry.
"The new Medical Devices Regulation is a major issue for our industry," Thomas Dietrich, Managing Director of the IVAM Microtechnology Network, explains. "It puts a heavy burden on manufacturers of components for the medical devices industry, and some have already announced that they will drop out of this business."
A tenth of the representatives of the microtechnology industry are involved in relevant decision making processes on EU level – with limited effects. Even experts with a long experience in Brussels describe decision making processes as complex and non-transparent, personal involvement as not efficient. Relevant impact, according to the involved industry representatives, can only be achieved through associations or national representatives.
"This is why IVAM will make effort to represent the interests of high-tech companies in the discussion of EU regulations in the future", says Thomas Dietrich. "And we will also campaign for an SME-friendly implementation of the Medical Device Regulation in Germany."