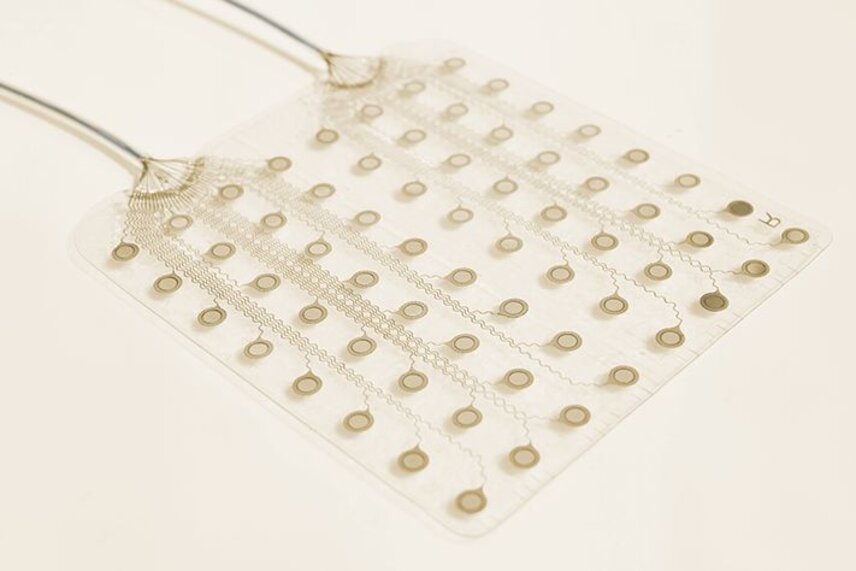
The AirRay Cortical Electrode is CorTec’s first medical device to receive market clearance from the Food and Drug Administration (FDA) in the USA for invasive neuromonitoring in the central nervous system. It can now be used as a diagnostic aid, for example before brain surgery. “For us as a company, the market clearance of our AirRay electrodes is an important milestone,” said Dr. Jörn Rickert, Chief Executive Officer of CorTec
Neuromonitoring ‘maps’ the brain prior to surgery
Some brain diseases may require surgical resection of the affected parts of the brain. AirRay Cortical Electrode is used for invasive neuromonitoring which comprises recording and stimulation of brain activity from the cortical surface over a period of time. In order to precisely locate diseased brain tissue such as epileptogenic foci or brain tumors while protecting healthy areas responsible for important brain functions, the brain must be ‘mapped’ prior to surgery. The electrode may be used for up to 29 days. CorTec introduces several innovations into this long-established method of treatment. The planar grid and strip electrodes are produced in a proprietary laser-assisted manufacturing process. This results in a very soft, thin, and flexible electrode that adapts well to the curved surface of the brain.
More precise diagnostics and shorter surgery durations with regard to brain diseases
In combination with new analytical methods AirRay Cortical Electrode may enable shorter surgery durations and more precise diagnostics in the future: For example with the aid of automated mapping of the brain in real-time, as enabled by the CortiQ system from g.tec (Austria), it will be possible to locate functional brain areas faster and more reliably in the future. A research group led by Nuri F. lnce at the University of Houston, Texas, is investigating high-frequency oscillations in brain activity as biomarkers for a more precise determination of epileptogenic foci. “Regulatory approval of CorTec’s AirRay Cortical Electrode opens up exciting new options both in clinical applications and in research using intracranial recording and stimulation methods,” commented neuroscientist Dr. Gerwin Schalk, Chair of the International Workshop on Advances in Electrocorticography series and Deputy Director of the National Center for Adaptive Neurotechnologies in Albany.
The product also contains important safety features. “Health and well-being of the patient are of the utmost importance to us. Therefore, we emphasize safe electrode design,” said Dr. Martin Schüttler, Chief Technology Officer and also Chief Executive Officer of CorTec. °AirRay Cortical Electrode has an even surface with individual electrode contacts almost being impalpable. Additionally, to prevent the contacts from dislocating or detaching from the silicone carrier material each contact safely interlocks with the material.
CorTec plans to market the AirRay Cortical Electrode through distribution partners. Corresponding contracts are currently in preparation
Contact: Dr. Christina Schwartz, CorTec GmbH
info@cortec-neuro.com